Join World's Fastest Growing B2B Network
预期用途
TheStrongStep
®
SARS-CoV-2
抗原快速检测是一种快速免疫层析法,用于检测人喉咙/鼻咽拭子中针对 SARS-CoV-2 病毒的
COVID-19 抗原。该检测方法用于帮助诊断
COVID-19。
引言
新型冠状病毒属于β属。COVID-19
是一种急性呼吸道传染病。人们普遍易感。目前,感染新型冠状病毒的患者是主要传染源;无症状感染者也可能是传染源。根据目前的流行病学调查,潜伏期为1~14天,多为3~7天。主要表现为发热、乏力和干咳。少数病例出现鼻塞、流鼻涕、喉咙痛、肌痛和腹泻。
原理
The
StrongStep ® SARS-CoV-2
Antigen Test employs chromatographic lateral flow
test device in a cassette format. Latex conjugated
antibody (Latex-Ab) corresponding to SARS- CoV-2 are
dry-immobilized at the end of nitrocellulose membrane
strip. SARS-CoV-2 antibodies are bond at the Test
Zone (T) and Biotin-BSA are bond at the Control Zone
(C). When the sample is added, it migrates by
capillary diffusion rehydrating the latex conjugate.
If present in sample, SARS-CoV- 2 antigens will bind
with the least conjugated antibodies forming
particles. These particles will continue to migrate
along the strip until the Test Zone (T) where they
are captured by SARS-CoV-2 antibodies generating a
visible red line. If there are no anti- SARS-CoV-2
antigens in sample, no red line is formed in the Test
Zone(T). The streptavidin conjugate will continue to
migrate alone until it is captured in the Control
Zone(C) by the Biotin-BSA aggregating in a line,
which indicates the validity of the test.
KIT COMPONENTS
|
20 Individually packed test devices |
Each device contains a strip with colored conjugates and reactive reagents pre-spreaded at the corresponding reqions. |
|
2 Extraction Buffer vials |
0.1 M Phosphate buffered saline (P8S) and0.02% sodium azide. |
|
20 Extraction tubes |
For specimens preparation use. |
|
1 Workstation |
Place for holding buffer vials and tubes. |
|
1 Package insert |
For operation instruction. |
MATERIALS REQUIRED BUT NOT PROVIDED
| Timer | For timing use. |
| Throat/Nasopharyngeal swab | For specimen collection |
PRECAUTIONS
This kit is for IN VITRO diagnostic use
only.
Read the instructions carefully before performing the
test.
This product does not contain any human source
materials.
Do not use kit contents after the expiration
date.
Handle all specimens as potentially
infectious.
Follow standard Lab procedure and biosafety
guidelines for handling and disposal of potentially
infective material. When the assay procedure is
complete, dispose specimens after autoclaving them at
121℃ for at least 20 minutes. Alternatively, they can
be treated with0.5% Sodium Hypochlorite four hours
before disposal.
Do not pipette reagent by mouth and no smoking or
eating while performing assays.
Wear gloves during the whole procedure.
STORAGE AND
STABILITY
The sealed pouches in the test kit may be stored
between 2- 30℃ for the duration of the shelf life as
indicated on the pouch.
SPECIMEN
COLLECTION AND STORAGE
Nasopharyngeal Swab Sample: It is important to obtain
as much secretion as possible. Therefore, to collect
a Nasopharyngeal Swab sample, carefully insert the
sterile Swab into the nostril that presents the most
secretions under visual inspection. Keep the Swab
near the septum floor of the nose while gently
pushing the Swab into the posterior nasopharynx.
Rotate the Swab several times. Throat swab: Depress
the tongue with a tongue blade or spoon. When
swabbing the throat, be careful not to touch the
tongue, sides or top of the mouth with the Swab. Rub
the Swab on the back of the throat, on the tonsils
and in any other area where there is redness,
inflammation or pus. Use rayon tipped swabs to
collect specimens. Do not use calcium alginate,
cotton tipped or wooden shaft swabs.
It is recommended that swab specimens be processed as
soon as possible after collection. Swabs can be held
in any clean, dry plastic tube or sleeve up to 72
hours at room temperature (15°C to 30°C), or
refrigerated (2°C to 8°C) before processing.
PROCEDURE
Bring tests, specimens, buffer and/or controls to
room temperature (15-30°C) before
use.
1. Place a clean Extraction tube in the designated
area of the workstation. Add 10 drops of Extraction
Buffer to the extraction tube.
2. Put the specimen swab into the tube. Vigorously
mix the solution by rotating the swab force fully
against the side of the tube for at least ten times
(while submerged).Best results are obtained when the
specimen is vigorously mixed in the solution. Allow
the swab to soak in the Extraction Buffer for one
minute prior to the next Step.
3. Squeeze out as much liquid as possible from the
swab by pinching the side of the flexible extraction
tube as the swab is removed. At least 1/2 of the
sample buffer solution must remain in the tube for
adequate capillary migration to occur. Put the cap
onto the extracted tube. Discard the swab in a
suitable biohazardous waste
container.
4. The specimens extracted can retain at room
temperature for 60 minutes without affecting the
result of the test.
5. Remove the test from its sealed pouch, and place
it on a clean, level surface. Label the device with
patient or control identification. To obtain a best
result, the assay should be performed within one
hour.
6. Add 3 drops (approximately 100 µL) of extracted
sample from the Extraction Tube to the sample well on
the test cassette. Avoid trapping air bubbles in the
specimen well (S), and do not drop any solution in
observation window. As the test begins to work, you
will see color move across the
membrane.
7. Wait for the colored band(s) to appear. The result
should be read at 15 minutes.
Do not interpret the result after 20 minutes. Discard used test tubes and Test Cassettes in suitable biohazardous waste container.
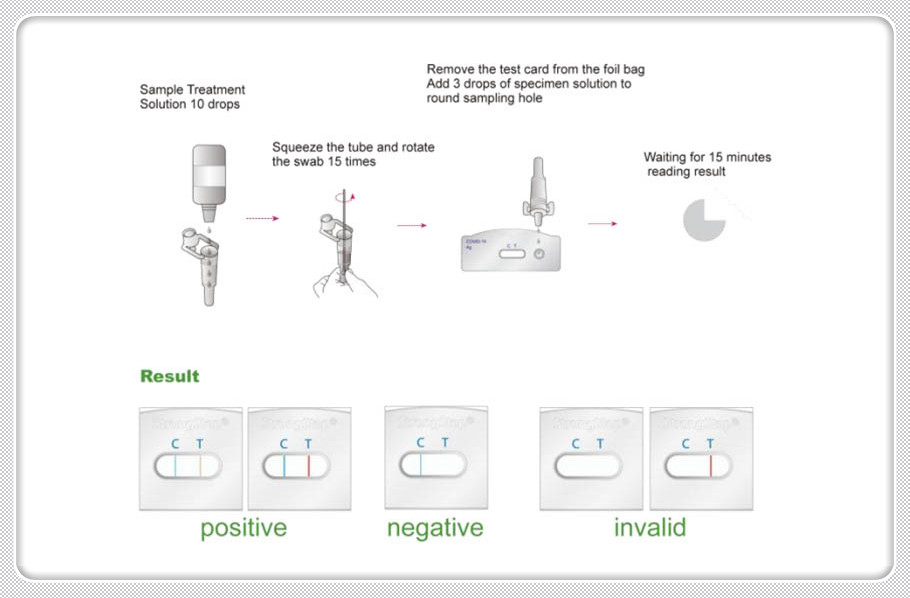
INTERPRETATION OF RESULTS
POSITIVE RESULT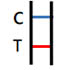
|
Two colored bands appear within 15minutes. One colored band appears in the Control Zone (C) and another colored band appears in the Test Zone (T). The test result is positive and valid. No matter how faint the colored band appears in the Test Zone (T), the test result should be considered a s positive result. |
NEGATIVE RESULT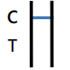
|
One colored bands appears in the Control Zone (C) within 15 minutes. No colored band appears in the Test Zone (T). The test result is negative and valid. |
INVALID RESULT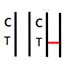
|
No colored band appears in the Control Zone (C) within 15 minutes. The test result is invalid. Repeat the test with a new test device. |
本试验的局限性1.
本试验用于人喉/鼻咽拭子样本中抗 SARS-CoV-2
抗原的定性检测,不注明抗原数量。
2.
该测试仅供体外诊断使用。
3.
与所有诊断测试一样,明确的临床诊断不应基于单一测试的结果,而应在评估所有临床发现后做出,尤其是结合
SARS-CoV-2 PCR 测试。4.
由于样本质量差或恢复期疾病时间点等原因,RT-PCR 检测 COVID-19 诊断的灵敏度仅为
30%-80%。SARS-CoV-2
抗原快速检测装置的灵敏度为理论上较低,因为它的方法论。
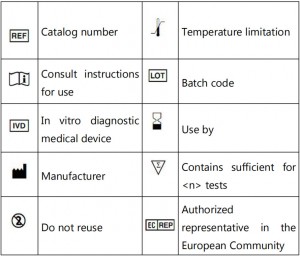
符号表