Join World's Fastest Growing B2B Network
5R MED instruments(CHENGDU)Co.,Ltd. was established in October 2018 and is located at No. 9, District 1, No. 618, West Section of Kelin Road, Chengdu Cross-Strait Science and Technology Industrial Development Park, Wenjiang District, Chengdu. It has a R&D and production base of 50,000 square meters, including a clean workshop of 3,000 square meters.

In February 2020, 5R MED engage in research and produce medical nitrile examination gloves, medical R&D, production, domestic sales and export of medical equipment such as PVC testing gloves, medical masks, FFP2 masks, KN95 masks, anti-fog protective goggles through the integration and reorganization of advantageous resources with China, European and American standard, in response to the major needs of the country’s epidemic prevention and control, had led by experts in the Thousand Talents Program and the Rong Piao Program.

Nitrile inspection gloves are produced in Jiangsu Jiangyin branch, Sichuan Yibin branch and Shandong Weifang branch. At present, 10 production lines have been put into use normally, and 40 production lines are under construction. At the same time, the company cooperated with Amend Pharmaceutical Group to jointly invest in the construction of a rubber glove production base, and plans to build 150 production lines. The project has received strong support from the local government and Amend Pharmaceutical Group.
The company's products provide protection and support for the People's Liberation Army, the national, provincial and municipal health systems, and thousands of companies. Currently, the products are exported to Finland, Italy, Germany, France, the United States, Canada, Australia, Poland, Switzerland, Greece, Serbia and other countries, and the export earns foreign exchange and help European and American countries to fight the epidemic.

CE

EU-TYPE EXAMINATION CERTIFICATE Certificate N° 0075/3743/162/02/21/0302 Manufacturer : 5R MED Instruments (CHENGDU) Co.,Ltd Building 9, Section 1, No.618 of West Kelin Road, Chengdu-Straits Technology Industry Development Zone, Wenjiang District, Chengdu, Sichuan Province China Descrition PPE Type : protective glove against microorganisms risks Product reference: 5R-GVN Glove description : Disposable Nitrile Gloves Available sizes : 7/S 8/M 9/L 10/XL

FDA 510 (K)

Proprietary Name: Polymer patient examination glove Classification Name: POLYMERPATIENTEXAMINATION GLOVE 510(K) Number: K210776 Product Code: LZA Device Class: 1 Registered Establishment name: 5R MED Instruments(CHENGDU)Co,Ltd. Registered Establishment Number: 3016843930 Owner/Operator: 5R MED Instruments(CHENGDU)Co,Ltd. Owner/Operator Number: 10067375 Establishment Operations: Manufacturer

EN ISO:13485:2016

5R MED INSTRUMENTS(CHENGDU) CO., LTD. Building 9, Section 1,No.618 ofWest Kelin Road, Chengdu Cross-Straits Technology Industry Development Zone, Wenjiang District, Chengdu, Sichuan Province, 611130, P.R. China ISO 13485:2016 EN ISO 13485:2016 Design,Packaging and Distribution of Nitrile Examination Gloves, Vinyl/ Nitrile Examination Gloves, Vinyl Examination Gloves, Latex Examination Gloves; Design, Manufacture and Distribution of Non-sterile Medical Face Mask This certificate is valid from 9July 2021 until 8 July 2024 and remains valid subject to satisfactory survilance audits. Recertication auditdue a minimum of 60 days before the expiration date lssue 1. Certified since 9 July 2021

ISO 9001:2015

5R MED INSTRUMENTS (CHENGDU)CO.,LTD Business Registration Address: Building 9,Section 1,No.618 of WestKelin Road, Chengdu Cross-Straits Technology Industry Development Zone,Wenjang Distrit Sichuan Province, 611130,P.R. China Unified Social Credit Code 91510115MA6B4BP17J has been assessed and crtified as meeting the requirements of ISO 9001:2015 Design,Packaging and Distribution of Ntrile Examination Gloves,Vinyl/ Ntrile Examination Gloves,Vinyl Examination Gloves,Latex Examination Gloves; Design,Manufacture and Distribution of Non-sterile Medical Face Mask This certificate is valid from 9 July 2021until 8 July 2024 and remains valid subject to satisfactory surveillance audits. Recertification audit due a minimum of 60 days before the expiration date. lssue 1. Certified since 9 July 2021

VOC Certificate

SGS VERIFICATION OFEN455 CONDITIONAL COMPLIANCE No: QDHL2012012968MDC Applicant : Manufacturer: 5R MED INSTRUMENTS(CHENGDU) CO., LTD. BUILDING 9.SECTION1.NO.618 OF WEST KELIN ROAD.CHENGDU CRoss-STRAITS TECHNOLOGY INDUSTRY DEVELOPMENT ZONE, WENJIANG DISTRICT.CHENGDU,, SICHUAN PROVINCE,CHINA Product Description: DISPOSABLE GLOVES Style No: 5R-GVN Color: BLUE Test Standard: EN 455-2:2015 MEDICAL GLOVES FOR SINGLE USE-PART 2: REQUIRMENTS AND TESTING FOR PHYSiCAL PROPERTIES CLAUSE4.2,4.3,5.2,5.3 EN 455-3:2015 MEDICAL GLOVES FOR SINGLE USE-PART 1: REQUIRMENTS AND TESTING FOR BIOLOGICAL. EVALUATION CLAUSE 4.6
ROHS certificate

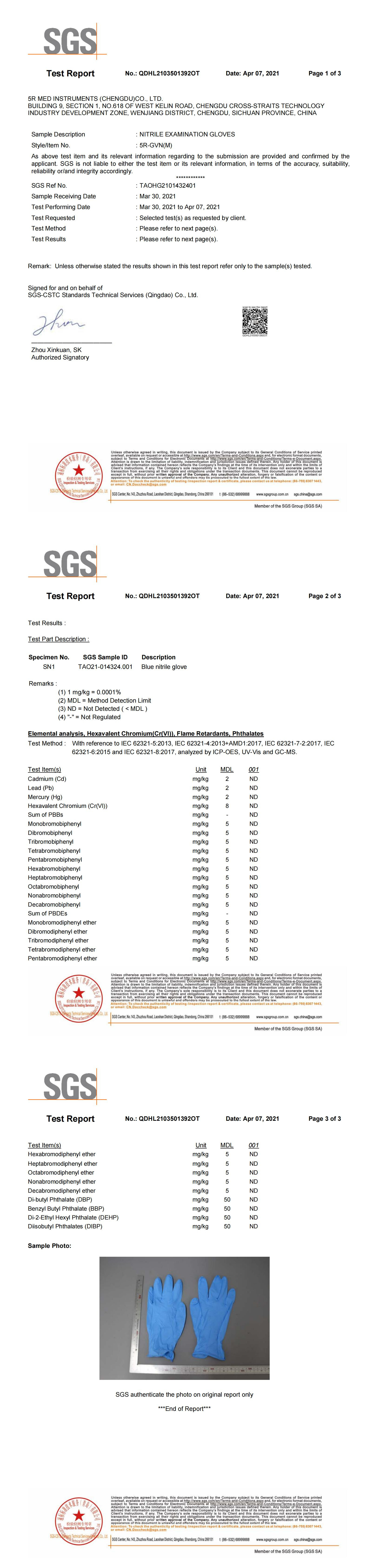
No.: QDHL2103501392OT Date: Apr 07, 2021 5R MED INSTRUMENTS (CHENGDU)CO., LTD. BUILDING 9.SECTION 1.NO.618 OFWEST KELIN ROAD.CHENGDU CROsS-STRAITS TECHNoLOGY INDUSTRY DEVELOPMENT ZONE.WENJIANG DISTRICT,CHENGDU, SiCHUAN PROVINCE,CHINA Sample Desciption : NITRILE EXAMINATION GLOVES Styel/ltem No.: 5R-GVN(M) SGS Ref No. : TAOHG2101432401: Mar 30, 2021 Sample Receiving Date : Mar 30, 2021 Test Performing Date : Mar 30, 2021 to Apr 07, 2021 Test Requested : Selected test(s) as requested by client.
| Business Type | Manufacturer |
| Company | 5R MED instruments(CHENGDU)Co.,Ltd |
| Main Products | Nitrile Glove,PVC Glove,Medical Face Mask,3ply Mask ,FFP2, |
| Website | Email: [email protected] |
| Established Year | 2018 |
| City / State | Chengdu , China |
| Country | China |
| Address | Building 9,Section 1,No.618 of West Kelin Road,Chengdu-Straits Technology Industry Development Zone,Wenjiang District,Chengdu, Sichuan Province, 611130 ,China |
| Location | Jiangsu Jiangyin branch, Sichuan Yibin branch and Shandong Weifang branch |
| Factory Size | 500,000 m2 In All |
| Total QC Staff | 20 |
| Total RND Staff | 24 |
| Number of Production Lines | 10 in production, 40 under construction |
| Annual output | 90-100 Million Box |
| Average Lead Time | 5 Days |
| Total Revenue | Above $ 10 Millions |
| Export Percentage | 100% |
| Nearest Port | Shanghai port(Ocean Shipping)/ Chengdu Port(Belt and Road) |
| Overseas Office |